Microtubule

Microtubules are one of the components of the cytoskeleton. They have a diameter of 25 nm and length varying from 200 nanometers to 25 micrometers. Microtubules serve as structural components within cells and are involved in many cellular processes including mitosis, cytokinesis, and vesicular transport.[2]
Contents |
Structure
Microtubules are polymers of α- and β-tubulin dimers. The tubulin dimers polymerize end to end in protofilaments. The protofilaments then bundle into hollow cylindrical filaments. Typically, the protofilaments arrange themselves in an imperfect helix with one turn of the helix containing 13 tubulin dimers each from a different protofilament. The image above illustrates a small section of microtubule, a few αβ dimers in length.
Another important feature of microtubule structure is polarity. Tubulin polymerizes end to end with the α subunit of one tubulin dimer contacting the β subunit of the next. Therefore, in a protofilament, one end will have the α subunit exposed while the other end will have the β subunit exposed. These ends are designated the (−) and (+) ends, respectively. The protofilaments bundle parallel to one another, so in a microtubule, there is one end, the (+) end, with only β subunits exposed while the other end, the (−) end, only has α subunits exposed. The (-) end is capped so elongation of the microtubule occurs from the (+) direction.
Organization within cells
Microtubules are nucleated and organized by the microtubule organizing centers (MTOCs), such as centrioles and basal bodies. Microtubules are part of a structural network (the cytoskeleton) within the cell's cytoplasm, but, in addition to structural support, microtubules take part in many other processes, as well. They are capable of growing and shrinking in order to generate force, and there are also motor proteins that allow organelles and other cellular factors to move along the microtubule. A notable structure involving microtubules is the mitotic spindle used by eukaryotic cells to segregate their chromosomes correctly during cell division. Microtubules are also part of the cilia and flagella of eukaryotic cells (prokaryote flagella are entirely different).
The process of mitosis is facilitated by a subgroup of microtubules known as astral microtubules, defined as a microtubule originating from the centrosome that does not connect to a kinetochore. Astral microtubules develop in the actin skeleton and interact with the cell cortex to aid in spindle orientation. They are organized into radial arrays around the centrosomes. The turn-over rate of this population of microtubules is higher than any other population. Astral microtubules function in concert with specialized dynein motors, which are oriented with the light chain portion attached to the cell membrane and the dynamic portion attached to the microtubule. This allows for dynein contraction to pull the centrosome towards the cell membrane, thus assisting in cytokinesis. Astral microtubules are not required for the progression of mitosis, but they are required to ensure the fidelity of the process; they are required for the correct positioning and orientation of the mitotic spindle apparatus. They are also involved in determination of cell division site based on the geometry and polarity of the cells. The maintenance of astral microtubules is dependent on the integrity of centrosome. It is also dependent on several microtubule-associated proteins such as EB1 and Adenomatous Polyposis Coli (APC).
Nucleation and growth
Polymerization of microtubules is nucleated in a microtubule organizing center. Contained within the MTOC is another type of tubulin, γ-tubulin, which is distinct from the α and β subunits which compose the microtubules themselves. The γ-tubulin combines with several other associated proteins to form a circular structure known as the "γ-tubulin ring complex." This complex acts as a scaffold for α/β tubulin dimers to begin polymerization; it acts as a cap of the (−) end while microtubule growth continues away from the MTOC in the (+) direction.
Dynamic instability
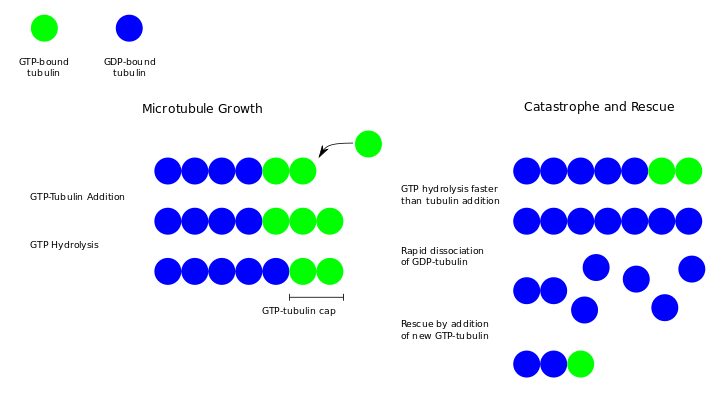
During polymerization, both the α- and β-subunits of the tubulin dimer are bound to a molecule of GTP. While the GTP bound to α-tubulin is stable, the GTP bound to β-tubulin may be hydrolyzed to GDP shortly after assembly. The kinetics of GDP-tubulin are different from those of GTP-tubulin; GDP-tubulin is prone to depolymerization. A GDP-bound tubulin subunit at the tip of a microtubule will fall off, though a GDP-bound tubulin in the middle of a microtubule cannot spontaneously pop out. Since tubulin adds onto the end of the microtubule only in the GTP-bound state, there is generally a cap of GTP-bound tubulin at the tip of the microtubule, protecting it from disassembly. When hydrolysis catches up to the tip of the microtubule, it begins a rapid depolymerization and shrinkage. This switch from growth to shrinking is called a catastrophe. GTP-bound tubulin can begin adding to the tip of the microtubule again, providing a new cap and protecting the microtubule from shrinking. This is referred to as rescue.[3]
In vivo microtubule dynamics vary considerably. Assembly, disassembly and catastrophe rates depend on which microtubule-associated proteins (MAPs) are present.
Chemical effects on microtubule dynamics
Microtubule dynamics can also be altered by drugs.
- For example, the taxane drug class (e.g. paclitaxel or docetaxel), used in the treatment of cancer, blocks dynamic instability by stabilizing GDP-bound tubulin in the microtubule. Thus, even when hydrolysis of GTP reaches the tip of the microtubule, there is no depolymerization and the microtubule does not shrink back.
- Nocodazole and Colchicine have the opposite effect, blocking the polymerization of tubulin into microtubules.
Motor proteins
In addition to movement generated by the dynamic instability of the microtubule itself, the fibers are substrates along which motor proteins can move. The major microtubule motor proteins are kinesin, which generally moves towards the (+) end of the microtubule, and dynein, which generally moves towards the (−) end.
Postulated role in consciousness
In their controversial Orch-OR theory of consciousness, Roger Penrose and Stuart Hameroff postulate that microtubules in neurons conduct quantum-level manipulations of matter which produces consciousness, based partially on widely recorded observations of Gamma Synchrony that indicate that information may propagate through the brain much faster than a chemically mediated neural network would physically permit. Max Tegmark disputes the relevance of these observations, and the matter remains open to debate.
Additional images
 Proteins in different cellular compartments and structures tagged with green fluorescent protein. |
References
- Manu Lopus, Mythili Yenjerla, and Leslie Wilson (2009) Microtubule Dynamics (Advanced Review). In Wiley Encyclopedia of Chemical Biology, Begley TP, Ed. Wiley, NJ. 3, 153–160.
- ↑ Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH (October 2002). "Microtubule structure at 8 Å resolution". Structure 10 (10): 1317–28. doi:10.1016/S0969-2126(02)00827-4. PMID 12377118.
- ↑ "Microtubule - Definitions from Dictionary.com". dictionary.reference.com. http://dictionary.reference.com/browse/Microtubule. Retrieved 2008-05-15.
- ↑ Mitchison T, Kirschner M (15-21 November 1984). "Dynamic instability of microtubule growth". Nature 312 (5991): 237–42. PMID 6504138.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||